Searching for Signs of Life in Space with Quantum Computers
Image credit: James Webb Space Telescope (NASA and STScI)
The results so far have been a promising validation of quantum’s potential to help us find life on distant planets.
Since the dawn of humanity, people have looked at the sky and wondered: are we alone?
Despite centuries of alleged UFO sightings and the concerted efforts of SETI, there is still zero convincing evidence that extraterrestrial life exists. However, that could soon change. Today, we are working with the University of Hull to add a new lens to the search for life: quantum computers.
Searching for Life in Deep Space
There are at least 100-200 billion — and likely as many as ten trillion — exoplanets just in our Milky Way galaxy. Even if you only consider planets with an orbit in the circumstellar habitable zone, or the “Goldilocks” zone around a star where a planet can support the conditions for life to flourish, there are still millions of planets that could plausibly support life.
Of course, most of these planets are thousands of light years away — it’s not like we can visit them to check for signs of life. Until we can, the only way we can search for life in space is to use our telescopes, including the recently launched James Webb Space Telescope (JWST).
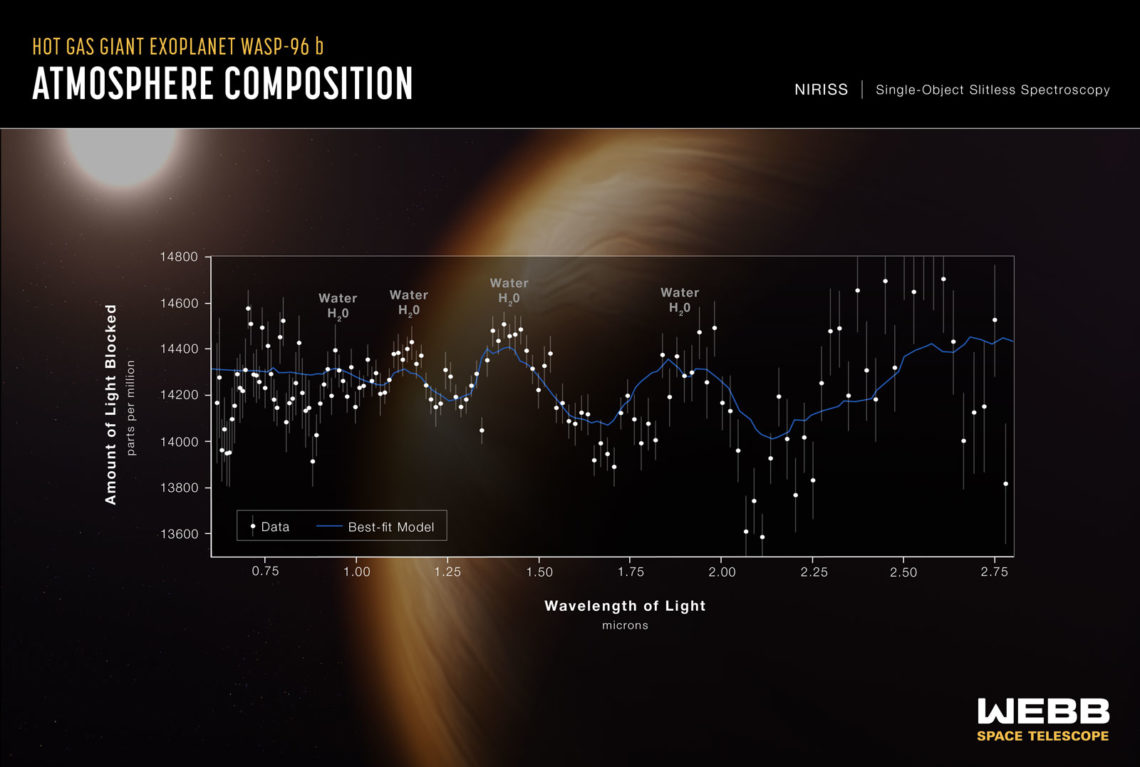
A spectroscopy analysis from JWST shows the presence of water on a distant planet. Credit: NASA
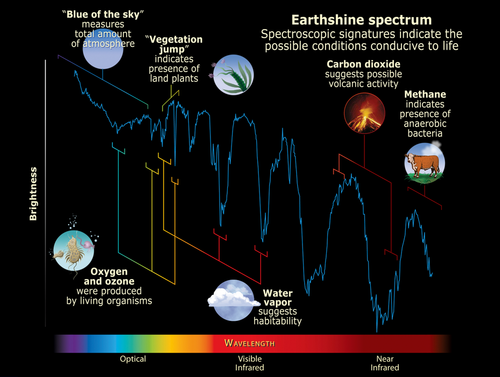
The JWST has captivated the world with its dazzling photographs of deep space. But in addition to capturing imagery in the optical light spectrum, it also captures cosmic infrared light with unprecedented fidelity. This infrared light can tell us a lot about the atmospheres on faraway planets. Every type of molecule absorbs a distinct pattern of infrared light frequencies, and by analyzing the missing frequencies in the light spectrum coming from a planet, we can tell which molecules are present in the atmosphere.
The presence of water, methane, ozone, ammonia, and various sulfur compounds in a planet’s atmosphere may all be indicative of life. But that’s just the tip of the iceberg. In 2016, MIT researchers published a database of over 16,000 molecules that could be signatures of life. However, we only have light signatures for about 300 of these molecules. To detect these signs of life across the galaxy, we need to understand how they interact with light.
Source: M. Turnbull & STScl
Quantum Computers Help Predict Signatures of Life
For two decades, researchers at the University of Hull have been using classical computers to predict the spectral signatures of molecules that could indicate the presence of life. This works OK with small molecules, such as a hydrogen molecule with only 2 electrons. But once you get to bigger molecules — even something relatively simple like CO2 — it can become too complex for even supercomputers to accurately simulate how a molecule’s electrons interact, which is essential to predicting the molecule’s spectral signature.
In principle, quantum computers are better suited to simulate molecules than classical computers. This is because quantum computers operate using quantum mechanics, the same laws that govern the behavior of molecules. Therefore, quantum computers may be able to calculate the molecular properties of interest without the massive computing power a classical simulation would require.
That’s why last year, the University of Hull teamed up with Zapata to develop quantum applications that could accurately simulate the molecular properties necessary to calculate a molecule’s spectral signature. Over the last several months, we have worked closely with the Hull researchers to port their existing quantum experiments from IBM Qiskit to Orquestra®, Zapata’s platform for building quantum-classical applications.
“By moving our quantum experiments from Qiskit to Orquestra,” said Dr. David Benoit, the lead researcher on the project from the University of Hull, “we’ve been able to reliably perform thousands of quantum simulations in a relatively short space of time. This was key to developing our initial idea into a workable approach.”
Unfortunately, we are still in the era of Noisy Intermediate-Stage Quantum (NISQ) devices, which are limited by their small numbers of qubits and high rates of error. However, Orquestra’s hardware agnostic format means that we can build application workflows to run on the noisy devices available today and plug in more powerful devices in the future — which will in turn enable us to simulate more complex molecules. Being hardware agnostic also allows us to experiment on different hardware backends today to evaluate which device will deliver the highest performance.
By moving our quantum experiments from Qiskit to Orquestra, we’ve been able to reliably perform thousands of quantum simulations in a relatively short space of time.
Dr. David Benoit, Lead Researcher at University of Hull
The Latest Updates from Our Research
To determine the spectral signature of a molecule, you first need to calculate the total energy of the molecule, also known as the Hamiltonian. The total energy can be broken down into two components: the mean-field energy and the electronic correlation. The mean-field energy refers to the energy of the electrons without accounting for the electronic correlation, which refers to the effects of the quantum mechanical interactions between the electrons.
Classical computers can easily calculate the mean-field energy. But calculating the electronic correlation is much harder and becomes exponentially more difficult to solve as the number of energy levels and the size of the molecule increases. It quickly reaches the point where no classical computer has enough memory to run these calculations, nor would they be able to complete the calculations on human time scales.
By exploiting their inherent use of quantum mechanics, quantum computers can estimate the electronic correlation using an algorithm known as the Variational Quantum Eigensolver (VQE), an algorithm pioneered by Zapata co-founder Alán Aspuru-Guzik for use on near-term quantum devices. Today’s noisy quantum devices, however, can only run VQE using a very small number of qubits. This is because the more qubits you use, the more error you generate, making the results increasingly inaccurate as you scale to larger molecules requiring more qubits. And when you’re looking at spectral signatures of molecules that are light years away, accuracy is extremely important.
Fortunately, we found a way to calculate the total energy of hydrogen using the quantum devices we have today. Our approach was to compute the exact electronic correlation using two simplified models of hydrogen, using two and six qubits respectively. From there, we extrapolated what the electronic correlation would be for a real hydrogen molecule, which would otherwise require 144 error-corrected qubits (which we don’t have). From there, we could calculate hydrogen’s spectral signature.
The results of this approach are significant. Even though we were using very few qubits, our results were indistinguishable from the results generated by the best-in-class classical solvers.
Now, hydrogen is still the simplest molecule in existence, and matching an existing classical solution may not seem like a breakthrough. After all, it has long been possible to detect hydrogen on another planet. Our breakthrough was in validating an approach that, as quantum hardware and error correction improve, may simulate more complex molecules beyond the reach of classical computing. With the workflow for this computation now built in Orquestra, we can easily swap in more powerful devices and error correction algorithms to simulate more complex molecules in the near future.
Now that we’ve proven our approach works with hydrogen, we will attempt to scale our approach to larger and more important molecules for life, such as water, methane, and ammonia.
Next Steps
The results so far have been a promising validation of quantum’s potential to help us find life on distant planets. Now that we’ve proven our approach works with hydrogen, we will attempt to scale our approach to larger and more important molecules for life, such as water, methane, and ammonia. There will certainly be more challenges with these more complex molecules, but our modular workflows on Orquestra will allow us to iterate on these calculations reliably and easily.
Furthermore, we’d like to test our approach on real quantum hardware. Our initial experiments were conducted on classical simulations of quantum computers. Using real hardware will confirm that our work is indeed scalable. In addition, we plan to apply propriety Zapata algorithms, such as robust amplitude estimation, to reduce the runtime in our calculations and make them more resistant to error, which will be critical as we scale to larger molecules.
As quantum hardware and error correction algorithms improve, we will be able to simulate more complex molecules and build a database of their spectral signatures. From there, we will be able to analyze the infrared data from the JWST to pinpoint planets that are likely to hold life. We may never know if this life is intelligent or at all like life on earth. But we will finally know that we are not alone.




